 Tom Greenbowe is an Emeritus Faculty member in the Department of Chemistry & Biochemistry at University of Oregon and a Morrill Professor of Chemistry Emeritus at Iowa State University. Greenbowe taught in the general chemistry program at UO for ten years. Prior to his move to Oregon he worked in the ISU general chemistry program for 25 years and was the Coordinator of General Chemistry. Tom has been involved with the AP Chemistry program and the College Board since 2007. Tom has served as a Table Leader and Question Leader for the AP Chemistry Exam from 2008 to 2024. Tom was appointed to AP Chemistry Test Development Committee from 2008 to 2012. In 2012 and in 2019 Tom attended AP Chemistry Professional Development Workshops that provided training and materials focusing on the new AP Chemistry framework and curriculum and the use of guided-inquiry in the classroom.
Throughout his teaching career Tom has integrated computers into his teaching. In 2005 Tom Greenbowe and Profs. John Gelder from Oklahoma State University and Michael Abraham from the University of Oklahoma received an NSF grant to a new technology project (http://genchem1.chem.okstate.edu/BDA/Topics.php) that provides activities before class, during class and after class using a learning cycle design to help student better understand introductory chemistry content. Participants in this workshop will have access to an APSI web site that includes homework assignments (with answers), sample examinations (with answers) and additional activities that can be incorporated into the classroom, as well as access to the GAG (Gelder-Abraham-Greenbowe) computer simulations URLs.
Tom Greenbowe and John Gelder worked with the ACS AACT to develop and produce an up-date to the “Metals and Metal Ions Activity” (aka Activity Series of Metals), and two electrochemical cells “Galvanic Cells” computer simulations in CSS/JAVA/HTM5. These simulations are currently being used by over 5,000 chemistry teachers.
Tom Greenbowe has been recognized for his contributions to the teaching and learning of chemistry through several awards.
Tom Greenbowe is an Emeritus Faculty member in the Department of Chemistry & Biochemistry at University of Oregon and a Morrill Professor of Chemistry Emeritus at Iowa State University. Greenbowe taught in the general chemistry program at UO for ten years. Prior to his move to Oregon he worked in the ISU general chemistry program for 25 years and was the Coordinator of General Chemistry. Tom has been involved with the AP Chemistry program and the College Board since 2007. Tom has served as a Table Leader and Question Leader for the AP Chemistry Exam from 2008 to 2024. Tom was appointed to AP Chemistry Test Development Committee from 2008 to 2012. In 2012 and in 2019 Tom attended AP Chemistry Professional Development Workshops that provided training and materials focusing on the new AP Chemistry framework and curriculum and the use of guided-inquiry in the classroom.
Throughout his teaching career Tom has integrated computers into his teaching. In 2005 Tom Greenbowe and Profs. John Gelder from Oklahoma State University and Michael Abraham from the University of Oklahoma received an NSF grant to a new technology project (http://genchem1.chem.okstate.edu/BDA/Topics.php) that provides activities before class, during class and after class using a learning cycle design to help student better understand introductory chemistry content. Participants in this workshop will have access to an APSI web site that includes homework assignments (with answers), sample examinations (with answers) and additional activities that can be incorporated into the classroom, as well as access to the GAG (Gelder-Abraham-Greenbowe) computer simulations URLs.
Tom Greenbowe and John Gelder worked with the ACS AACT to develop and produce an up-date to the “Metals and Metal Ions Activity” (aka Activity Series of Metals), and two electrochemical cells “Galvanic Cells” computer simulations in CSS/JAVA/HTM5. These simulations are currently being used by over 5,000 chemistry teachers.
Tom Greenbowe has been recognized for his contributions to the teaching and learning of chemistry through several awards.
Course Description Part 1
Welcome to the AP® Chemistry Summer Workshop as presented by Marian DeWane and Tom Greenbowe. One goal of the workshop is to provide each participant an opportunity to gain a deeper understanding of how the AP® Chemistry topics and learning objectives govern the AP Chemistry curriculum and the AP Chemistry Test. Chemistry content topics planned for discussion include: Stoichiometry, Thermochemistry (calorimetry), Photoelectron Spectroscopy, Maxwell-Boltzman Distribution, Intermolecular Forces, Kinetics, Acid-Base Equilibria, Energetics of Solution Formation, Thermodynamics and Electrochemistry (Galvanic and Electrolytic Cells). Other topics can be substituted depending on the needs of the participants. Participants will work through five laboratory experiments using a combination of two guided-inquiry approaches: the Science Writing Heuristic (SWH) and Process Oriented Guided Inquiry Learning (POGIL). Participants will learn about the SWH and how it supports both students and teachers doing guided-inquiry laboratory experiments. For each laboratory activity participants will construct a ‘Research Question’ and design an experiment to answer the question, write claims and evidence statements and write an effective discussion about the experiment. Why attend an in-person APSI? Meaningful discussions and collaborations! Opportunities to do hands-on activities and laboratory experiments.
Participants will experience doing guided-inquiry classroom activities, interactive demonstrations, and incorporating computer simulation and animations in their presentations. We will also modify POGIL activities by incorporating computer simulations and animations developed by John Gelder (Oklahoma State University), Mike Abraham (University of Oklahoma), and Tom Greenbowe (University of Oregon).
Click here to see our our simulation live
Why attend an in-person APSI? Meaningful discussions and collaborations! Opportunities to do hands-on activities and laboratory experiments.
Participants will experience doing guided-inquiry classroom activities, interactive demonstrations, and incorporating computer simulation and animations in their presentations. We will also modify POGIL activities by incorporating computer simulations and animations developed by John Gelder (Oklahoma State University), Mike Abraham (University of Oklahoma), and Tom Greenbowe (University of Oregon).
Click here to see our our simulation live
Sample URL: https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
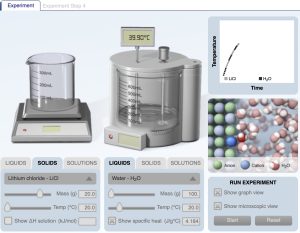
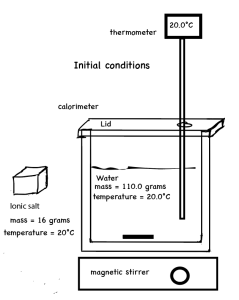
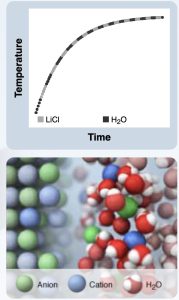
Calorimetry computer simulation dissolving lithium chloride in water. The calorimetry computer simulation provides a temperature versus time graph and an animation of the dissolving process at the particle level of representation.

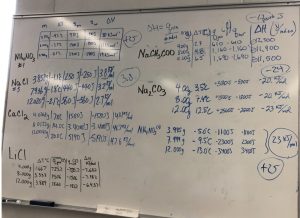
AP Chemistry teachers perform calorimetry experiments of dissolving various salts in water. The teachers pool data, observe trends, and determination the thermal energy transferred and determine the change in enthalpy for dissolving different salts in water. This is why we do hands-on calorimetry experiments.
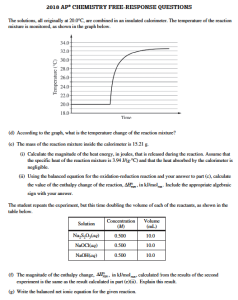
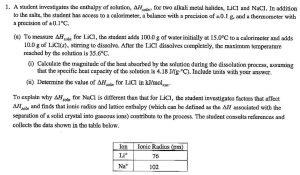
Having your students do a calorimetry experiment helps students gain a better understanding of the transfer of energy and leads to a good score on calorimetry free-response questions (or a multiple-choice questions) on an AP Chemistry Test. Interactive activities are designed to prepare your students to understand specific chemistry concepts and principles so that the students will be able to solve the problems on the APChem Test. This workshop will provide each participant with instructional resources to draw upon during the school year. Each participant will develop two instructional units for use in their AP Chemistry course.


