Course Description Part 2 (Agenda follows the course description)
Participants are encouraged to bring two existing classroom lessons, a demonstration, and two laboratory activities to share and to refine. Participants will work through six sample classroom activities using the Process Oriented Guided Inquiry Learning (POGIL) approach. We will work in teams to revise existing AP Chem classroom activities by incorporating (POGIL) principles.
Participants will be provided with digital access to the AP® Chemistry Workshop Handbook and with web-based access to a variety of preparatory materials for the AP® Chemistry course. Participants will “read” (score) sample student work from the free-response questions from the recent AP® Chem Test. As a group, we will explore the topics students exhibited difficulty understanding on the current and previous years’ AP® Chem Tests. Participants will learn strategies for helping students to understand what level of response is needed on the AP Chem Test with respect to writing effective justifications and explanations. We will review the test statistics for the current AP® Chem Test as well as select statistics for previous tests. As a group we will explore effective assessments and review the questions in the AP Chemistry Classroom Test Bank.
Participants have the option to share a favorite lesson, demonstration, laboratory activity, etc. Please bring an activity you want to share. Peer sharing will occur usually at the end of each day. Participants who volunteer to share activities are limited to a 10-minute presentation or a 15-20 minute hands-on activity.
What you need to bring to the workshop: Safety Goggles, a scientific calculator, a lab-top computer, notebook, pencil, eraser, your current AP® Chem course syllabus, your AP® Chem laboratory manual, and any instructional resources you may wish to share with the group such as a favorite laboratory experiment or favorite classroom activity. Participants should work the free-response problems on the current AP® Chem Tests prior to the workshop.
Laboratory Safety Note: appropriate attire is required each day when we are doing chemistry laboratory experiments. Please bring to the workshop: safety goggles, a pair of socks, closed toed shoes, a shirt with sleeves (can be short sleeves), long pants or a long dress, and safety goggles. A lab coat is optional.
Instructional Resources: During the workshop, participants will be invited to Marian and Tom’s AP Chemistry Google Drive. This Google Drive contains all of the AP Chem workshop files discussed at the APSI AP Chem workshops plus many additional instructional files. Participants will also be invited to use the public and “by invitation only to instructors ” instructional resources located on the new CIDER web-site – Chemistry Interactive Demonstrations and Educational Resources. https://cider.uoregon.edu/
The old site is “Chemdemos”. University of Oregon Chemistry Demonstrations & Chemistry Instructional Resources web site: https://chemdemos.uoregon.edu/

Agenda and Daily Schedule
TENTATIVE Workshop Schedule for a 4 or 4.5 Day Workshop
This schedule will change in accordance with the participant needs as determined during the first day of the workshop.
Day 1
- Introduction of participants
- Workshop Content and Objectives – Participants preferences will taken into account
- Groups work:Analyzing and summarizing the AP® Chemistry Topics, Science Practices and the Learning Objectives
- AP Topic Stoichiometry. An analysis of the quantity of water in a sample of copper(II)sulfate hexahydrate. Empirical Formula, Molecular Formula.
- AP Topic Thermochemistry: CalorimetryA POGIL classroom activity, a SWH laboratory activity, a calorimetry computer simulation, AP® Chem Test Free-Response Questions on Calorimetry.
- The AP® Chemistry Curriculum Framework:What instructors need to know:a. You do not have enough time to teach everything in the curriculum, b. There are topics in the textbook that are not assessed on the AP* Chemistry Test, c. You need to know some chemistry beyond what is in the general chemistry textbook, d. Even though you are teaching at a high school your AP® Chemistry course is expected to have the same things (topics, laboratory experiments, tests, etc.) that are in a college or university level General Chemistry course.
- Analysis and review of a thermochemistry question from the current AP® Chemistry Test.
- AP® Chemistry Test … all questions are tied to one or more of the AP Chem Learning Objectives.
- Assessing conceptual understanding using concept questions and particle (molecular view) diagrams.
- Reasoning from Data and models. The power of written explanations and justifications.
- The AP® Chemistry Laboratory Requirements focusing on guided-inquiry.
- Guided-Inquiry, Learning Cycles, the Science Writing Heuristic (SWH) and Process Oriented Guided Inquiry Learning (POGIL).Introduction to POGIL using a POGIL activity.
- A hands-on lesson of how the learning cycle, group work, pooling data, and POGIL/SWH drives instruction in the lab and in the classroom.
Day 2 (classroom, laboratory, and assessment)
AP® Chem Areas 1 and 2, and Corresponding Learning Objectives
Tentative Topics
- Intermolecular Forces
- Energetics of Solution Formation (lattice energy, heat of hydration, Born-Haber diagrams)
- Acid/Base Equilibrium (pH, ICE Tables, Ka, Kb, titrations, buffer solutions)
- The use of particle diagrams to represent atoms at the molecular level.The use of computer simulations and animations in the classroom and in the laboratory.
- Converting traditional (or verification) labs to inquiry laboratory activities.
- Converting traditional classroom lessons into inquiry lessons.
Day 3 (classroom, laboratory, and assessment)
AP® Chem Big Ideas 3 and 4, and Corresponding Learning Objectives
Tentative Topics
- Acid/Base Equilibrium
- Kinetics (rate laws, integrated rate equations, mechanisms)
- Electrochemistry
- Compacting the AP® Chem curriculum
- Characteristics of guided-inquiry instruction. How do you know a lesson is guided-inquiry?
- Submitting an AP® Chem audit – if you are new to the AP Chem program.
Day 4 and or Day 4.5 (classroom, laboratory, and assessment)
AP® Chem Big Ideas 5 and 6, and Corresponding Learning Objectives
Tentative Topics
- Electrochemistry: Galvanic Cells and Electrolysis Cells
- An Electrolysis computer simulation of a copper-copper cell affords the particle level representation of what occurs at the cathode and anode electrodes
- Thermodynamics: Gibbs Free Energy
- Participants will develop classroom & laboratory instructional units and assessment questions on specific topics and share with the group.
- Diversity, Inclusion and Equity in your AP classroom
- How to prepare your students to be successful on the AP® Chemistry Test.
- Wrap-Up and Evaluation.
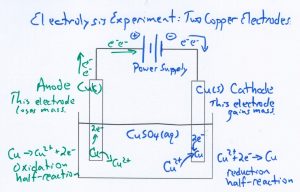
Electrolysis Experiment


http://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/html5/Electro/Electro.php



Electrolysis experiment investigating what influence varying the current has on the mass of copper metal produced. AP Chemistry teachers interact with an electrolysis computer simulation and a hands-on electrolysis experiment. Teachers vary the current, time and type of metal electrodes in an electrolysis circuit.

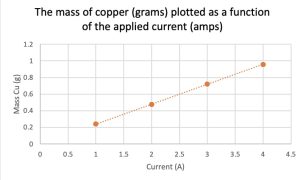
Electrolysis experiment investigating what influence varying the current and time has on the metal produced on the way to determining Faraday’s Constant.
This is why we do chemistry electrolysis experiments. Sample AP Chem Test question assessing knowledge of electrolysis.

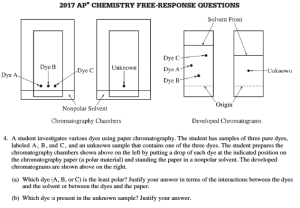
Paper Chromatography Experiment
AP Chemistry Teachers practice the 12 laboratory techniques necessary for good chromatography results.


Chromatography Results Using Different Solvents (polar vs non-polar)
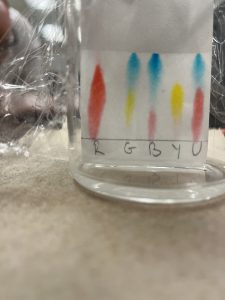

AP Chemistry Chromatography Test Question – This is Why We do Hands-On Chemistry Laboratory Experiments